[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion
Chemistry 101A Topic F: Molecular Structure 9: Basic Concepts of Covalent Bonding 9.3: Drawing Lewis Structures
Hco3lewis Structure
Lewis Structure of Carbonic Acid (H2CO3) The formula of carbonic acid is H2CO3. It has two H atoms, one C atom, and three O atoms. To understand the molecular formula of H2CO3, we have to observe the electronic configuration of the participating atoms and how many atoms they have in the outer shell.
[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion
Check me out: http://www.chemistnate.com
[Solved] Write Lewis structures for these ions. (a) HCO3^ Bicarbonate ion
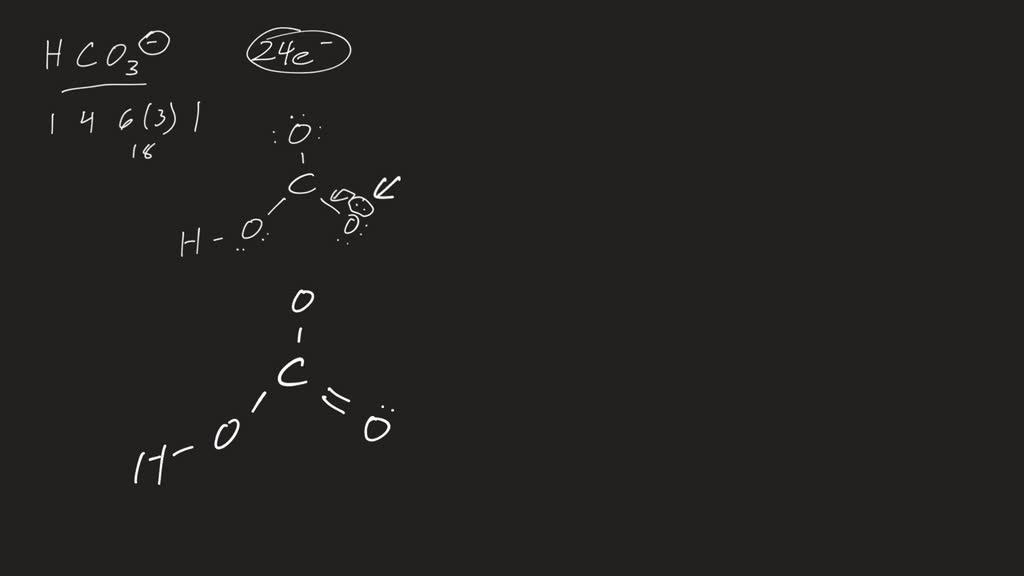
How to draw lewis structure of HCO3-? The bicarbonate (HCO3-) ion comprises a carbon (C) atom at the center. It is double-covalently bonded to an oxygen (O) atom at one side and to another O-atom and an OH functional group vis single covalent bonds at the other two sides. There is no lone pair of electrons on the central C-atom.

Lewis Dot Structure For Sodium
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
Lewis dot structure for hco3 examquiz
Frequency calculation at the HF/6-31G*//HF/6-31G* level showed that the structure 8 is not a minimum, as it contains two imaginary frequencies. $\ce{H3CO3+}$ shares structural similarities with its triaza-analog, the guanidinium ion, as both are possessing resonance stabilization via their onium forms [3, p. 60]. References

Lewis dot structure for hco3 examquiz
HCO3- Molecular Geometry / Shape and Bond Angles Wayne Breslyn 725K subscribers Join Subscribe Subscribed 29K views 10 years ago A quick explanation of the molecular geometry of HCO3- including.

Bicarbonate anion hco3 structural chemical Vector Image
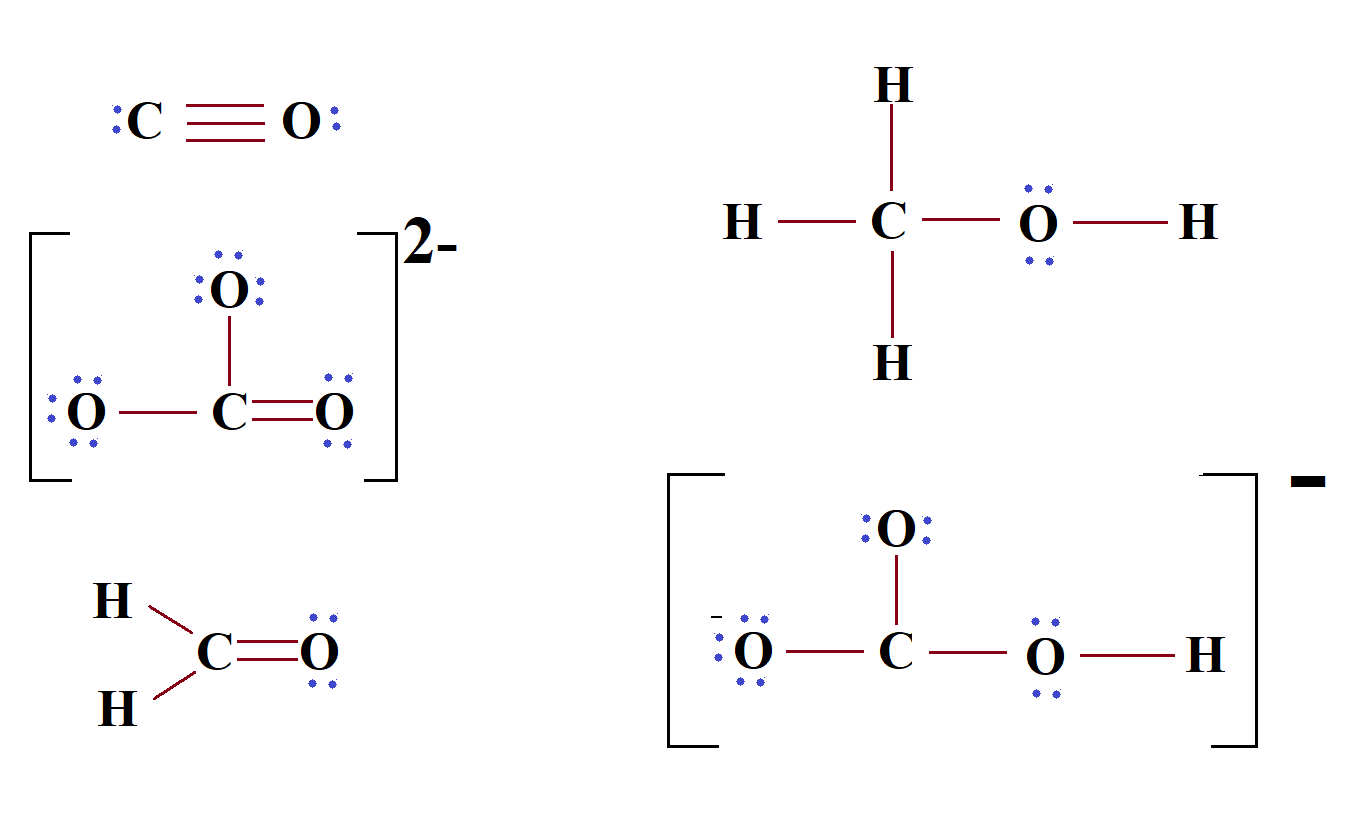
Bicarbonate (HCO3-) Ion Lewis Structure Iodate ion (HCO 3-) Ion Lewis Structure Bicarbonate ion contains one carbon atom, three oxygen atoms and one hydrogen atom. Lewis structure of carbonate ion (HCO 3-) contains one C=O bond, two C-O bonds and one O-H bond. There is -1 charge on one oxygen atom in HCO 3- lewis structure. HCO 3- lewis structure
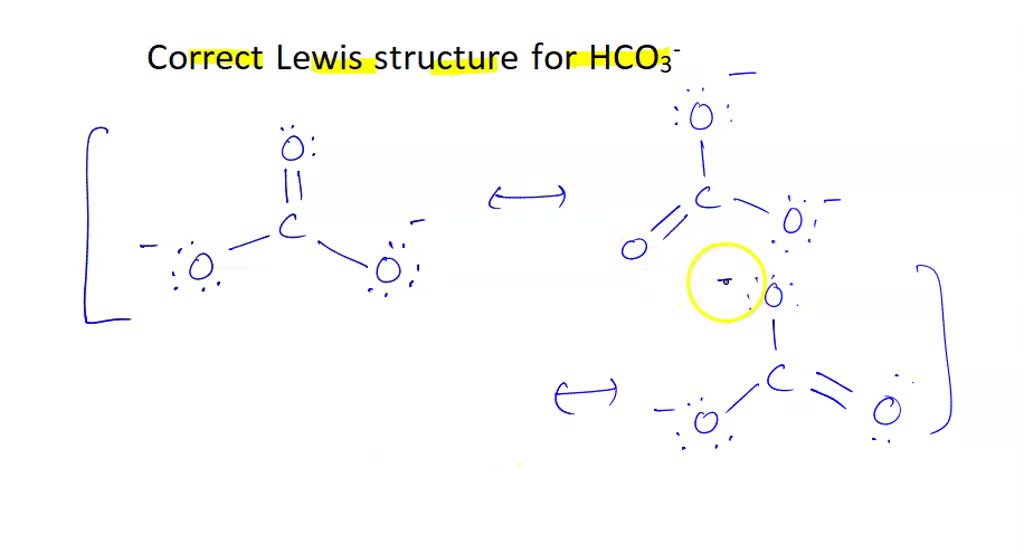
SOLVED Which of the following is the correct Lewis structure for HCO3
HCO3- Lewis structure November 7, 2023 by Deep The information on this page is fact-checked. HCO 3- Lewis structure HCO 3- (bicarbonate) has one hydrogen atom, one carbon atom, and three oxygen atoms. In the HCO 3- Lewis structure, there is one double bond and two single bonds around the carbon atom, with three oxygen atoms attached to it.
Lewis Structure For Hco3
HCO3- lewis structure has a Carbon atom (C) at the center which is surrounded by two Oxygen atoms (O) and one O-H group. There is 1 double bond between the Carbon atom (C) & Oxygen atom (O) and the rest other atoms have a single bond. There is a -1 formal charge on the single bonded Oxygen atom (O).
SOLVED(a) The structure of the bicarbonate (hydrogen carbonate) ion
A step-by-step explanation of how to draw the HCO3- Lewis Dot Structure (Hydrogen Carbonate or Bicarbonate Ion).For the HCO3- structure use the periodic tabl.

Hco3lewis Structure
Subscribe 661 views 1 year ago Lewis Structure Hello Guys! In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the.

HCO3 Lewis structure, Molecular geometry, Hybridization, Polar or
In the lewis structure of carbonic acid (H 2 CO 3 ), carbon atom is the center atom and there are two -OH groups. Also, there is one double bond between carbon and oxygen atoms. As some molecules. there are no lone pairs on carbon atom. From H 2 CO 3 lewis structure, we can say H 2 CO 3 is a dibasic acid. In this tutorial, we will cover how to.
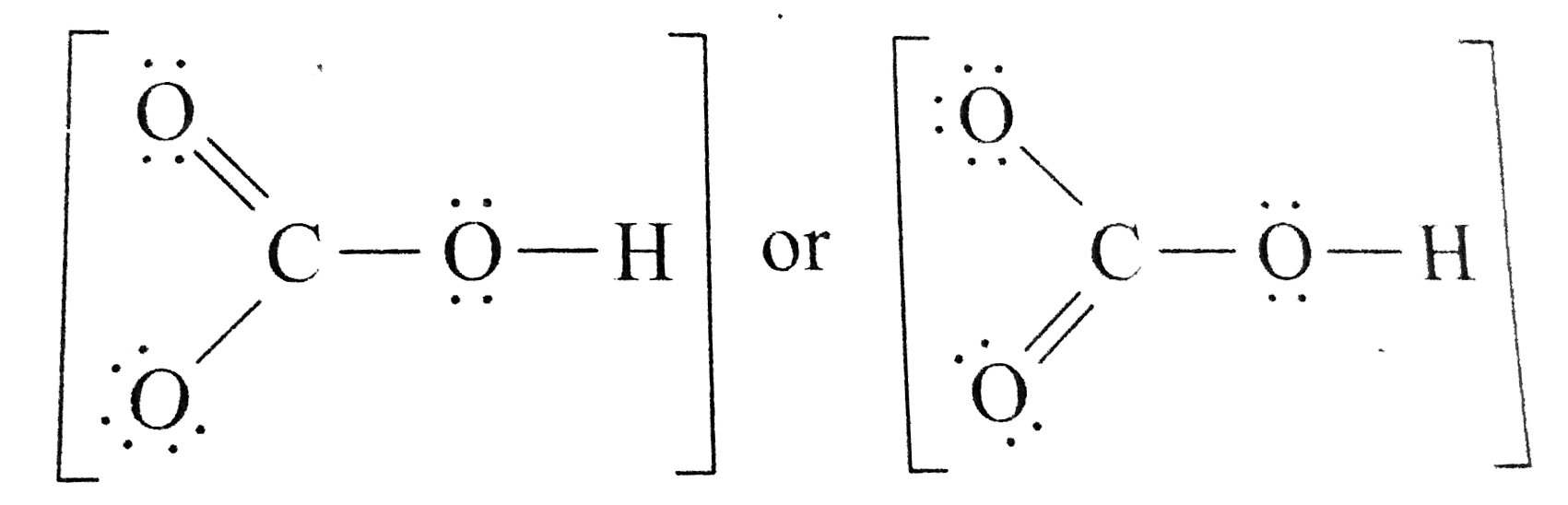
Draw a Lewis structure for the bicarbonate ion, HCO3^().
There are two resonance structures HCO3 - (Bicarbonate ion). We start with a valid Lewis structure and then follow these general rules. For the HCO3 - reson.
Hco3lewis Structure
You should put the H CO 3- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge. There are a total of 24 valence electrons in H CO 3-. HCO3- Lewis Structure: How to Draw the Lewis Structure for HCO3- Watch on See the Big List of Lewis Structures

HCO3 Molecular Geometry / Shape and Bond Angles YouTube
By Sarnali Mukherjee HCO3- Lewis structure is reliable in denoting considerable chemical and physical properties of Bicarbonate. As Lewis structure brings forth a fundamental sketch of HCO3-, it is effective in highlighting the electronic fact about the compound.